Abstract
Introduction Acute myeloid leukemia (AML) is a heterogeneous group of hematological malignancy. Classical induction therapy of DAE composed of etoposide (VP-16), cytarabine arabinoside (Ara-C) and anthracycline (DNR) is commonly applied in childhood AML, but etoposide has been reported to increase the risk of secondary cancer, especially for AML with MLL rearrangement. Although homoharringtonine (HHT) is commonly used for the treatment of Chinese adult AML, the efficacy and safety of HHT-based induction therapy have not been confirmed for childhood AML. And whether survival of pediatric AML will benefit from maintenance therapy without increasing toxicity is a matter of debate. All-trans retinoic acid (ATRA)-based therapy has been less reported and verified among non-APL AML patients. Here, we seek to evaluate the efficacy and safety of HHT-based induction regimens combined ATRA-based maintenance therapy for Chinese childhood AML (Chinese Clinical Trial Registry: ChiCTR-IPR-15006816).
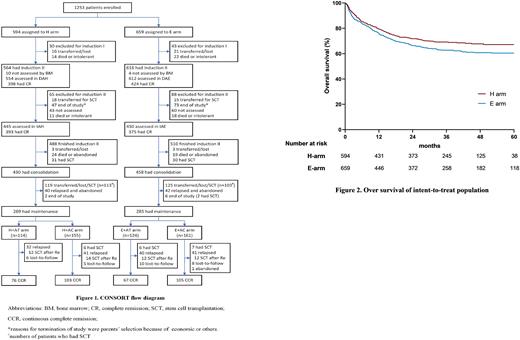
Methods The 2015 open-label, multicenter, randomized Chinese Children's Leukemia Group (CCLG) study was performed across 35 centers in China between July 2015 and October 2019. Newly diagnosed 0- to 18-year-old children with AML were first randomly assigned to receive HHT-based (H arm) or etoposide-based (E arm) induction regimens [HHT (3 mg/m2 per day from days 1 to 5) or VP-16 (100 mg/m2 per day from days 1 to 5) combined with DA regimen (D: 40 mg/m2 per day on days 1, 3 and 5; A: 100 mg/m2 every 12 hours from day 1 to 7)] and then randomly allocated to receive cytarabine-based (AC arm) or ATRA-based (AT arm) maintenance therapy. The primary endpoint is the complete remission (CR) rate after two courses of induction therapy between H arm and E arm. The secondary endpoints include the 3-year overall survival (OS) and 3-year event-free survival (EFS) of patients in the intent-to-treat population among four arms (H+AT arm, H+AC arm, E+AT arm and E+AC arm). What we should explained is that the study was initially designed as a multicenter randomized controlled trial, however, due to a shortage of HHT, which lasted for half a year in some local hospitals, we had to give priority to ensuring patient benefit and assign these patients to the E arm until HHT became available, and then patients in the H arm were supplemented in those hospitals. So patients were unevenly distributed among the four arms.
Results We enrolled 1253 intent-to-treat de novo AML patients, of whom 895 were included in the induction therapy analysis, and 554 received maintenance therapy (Figure 1). The median age was 75.0 months (IQR 36.0-118.0), and 57% of the patients were male. The median follow-up time was 29.4 months (IQR 8.4-47.4). 185 of 1253 patients (14.8%) were lost to follow-up, of whom 70.0% patients have been followed for over 12 months. The CR rates were 88.3% in the H arm versus 83.3% in the E arm (p=0.03). The CR rate of patients with t(8;21)/AML1-ETO was higher in the H arm than in the E arm (94.7% vs. 87.5%, p=0.018). According to the intention-to-treat analysis, the 3-year OS and EFS were significantly higher in the H arm than in the E arm [3-year OS: 69.2% vs. 62.8%, p=0.025 (Figure 2); 3-year EFS 61.1% vs. 53.4%, p=0.022]. Among patients who were enrolled in maintenance therapy (per-protocol population), the 3-year EFS (H+AT arm: 70.7%, 95% CI 61.1-78.3; H+AC arm: 74.8%, 95% CI 67.0-81.0, p=0.933; E+AC arm: 72.9%, 95% CI 65.1-79.2, p=0.789; E+AT arm: 66.2%, 95% CI 56.8-74.0, p=0.336) did not differ significantly across the four arms. In subgroup analysis, patients with AML1-ETO positivity in the H+AT arm had significantly better OS (82.4% vs. 66.4%, p=0.043) and EFS (73.6% vs. 52.8%, p=0.013) than those in the E+AC arm.
Conclusions Our study demonstrated, in childhood AML, HHT-based induction regimens (DAH) could significantly obtain higher complete remission rate compared with patients who received etoposide-based regimens, especially for those with AML1-ETO positive. And The HTT-based regimen resulted in a significantly improved 3-year OS and EFS compared with VP-16-based regimen. More patients with AML1-ETO positive who took DAH achieved complete remission, and these patients had longer overall survival and event-free survival, than those who took DAE. And ATRA-based maintenance has comparable effects to cytarabine-based maintenance in terms of OS and EFS. ATRA may be an alternative option for pediatric AML.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal